Discover What’s Next in Neuroimmunology Drug Development
The 8th Neuroimmunology Drug Development Summit is the definitive meeting uniting the brightest minds in pharma, biotech, and academia to accelerate the next generation of immune-modulating therapies for neurodegenerative, neuropsychiatric, and inflammatory CNS disorders.
With breakthroughs in glial biology, peripheral immune pathways, and advanced omics technologies, the field is beginning to decode the mechanisms driving neuroinflammation and unlock fresh therapeutic opportunities beyond established targets like TREM2, NLRP3, and BTK.
Across four immersive workshop sessions and three days of cutting-edge content, you’ll explore how peripheral-central immune interactions, emerging biomarkers, and innovative preclinical models are reshaping how CNS disorders are understood, targeted, and treated.
Expect an agenda packed with actionable insights, interactive discussions, and unparalleled networking opportunities with 80+ senior discovery, preclinical, and translational leaders from Sanofi, Biogen, Pfizer, Merck, Muna Therapeutics, Lundbeck, AbbVie, and more.
Join this exclusive community of scientific and strategic pioneers and be part of the movement transforming neuroimmunology R&D and advancing therapies for patients in urgent need.

What's New for 2026
New Industry Leaders Presenting
2026 brings together innovative biotech leaders such as Neils Plath of Muna Therapeutics and Ralph Minter of Alchemab, along with fresh academic research from institutions such as the Broad Institute and Mount Sinai.
Unique Focus on Targeting the Peripheral Immune System
Exploring B and T cell targets, fresh insights into interplay between adaptive and innate immunity immunity, and combined anti-inflammatory and neuroprotective mechanisms with Immunic Therapeutics and Bristol Myers Squibb.
New Deep-Dive on Inflammatory Drivers of Psychosis
Featuring breakthrough insights from the Broad Institute redefining psychiatric–neurological boundaries and informing the next generation of psychosis treatments.
Preclinical & Translational Strategy Debate
Hear candid discussions on the potential post animal model era, humanized and chimeric systems, and smarter target-driven design to improve translational confidence in neuroimmune drug development with Bristol Myers Squibb, Biogen and Merck.
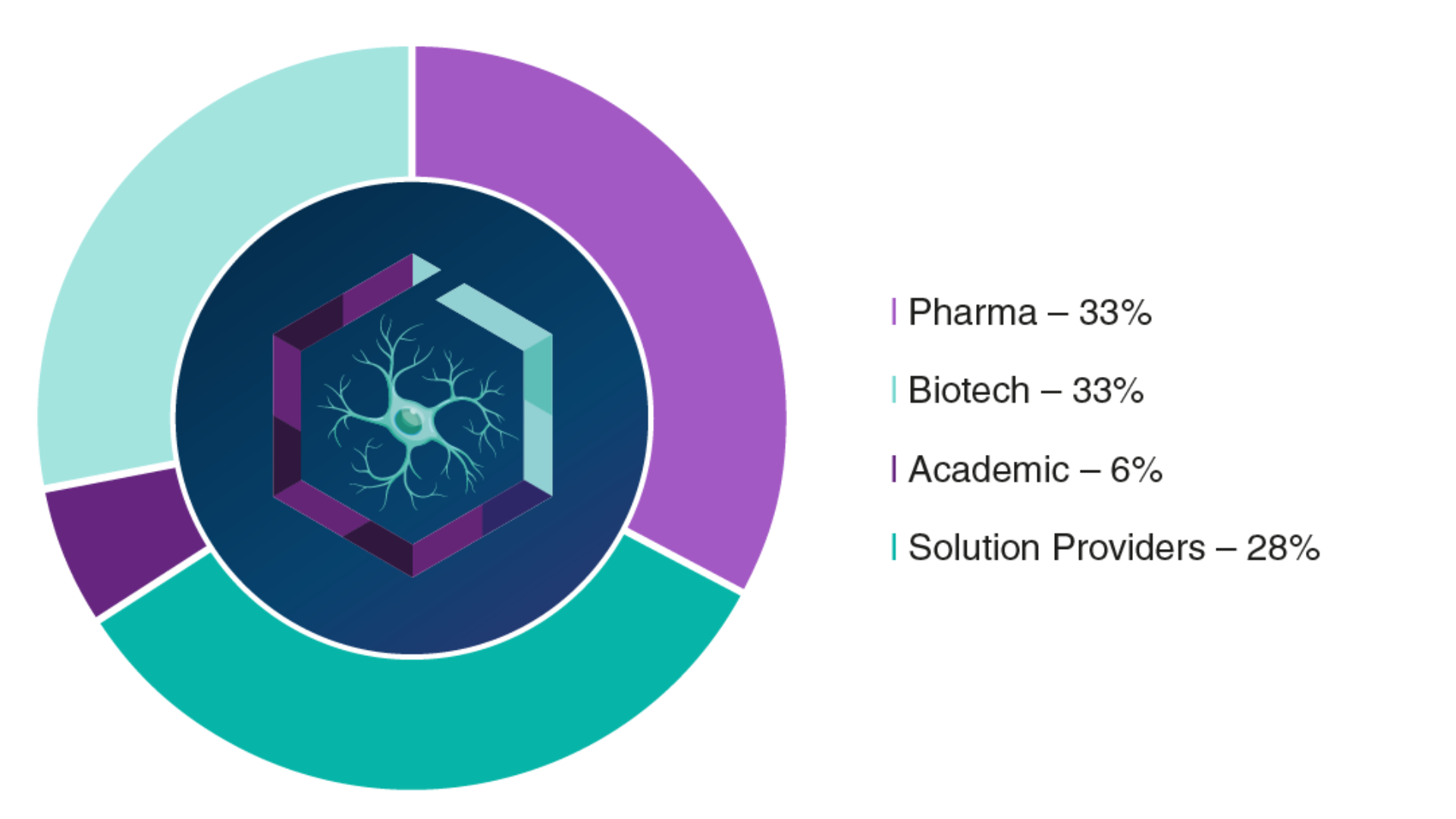
Audience Composition
Company Type
Attendee Seniority

Divide & Conquer
Built with insights from leading experts, the pre-conference program has been developed to tackle the key innovations and challenges at different stages of neuroimmunology drug development. The exciting pre-conference day features two tailored tracks:
- Track A – Target Discovery: Focused on uncovering novel neuroinflammatory mechanisms and actionable targets in neurodegenerative psychosis and mood disorders.
- Track B – Translational Tools: Dedicated to bridging preclinical research and clinical translation with cutting-edge models, biomarkers, and strategies
Neuroinflammation & Neuroimmune Pathways in Early-Onset & Neurodegenerative Psychosis
Explore how inflammation drives psychosis across Alzheimer’s, autoimmune encephalitis, and anti-NMDA receptor syndromes, redefining psychiatric–neurological boundaries.
Workshop Lead: Matthew Johnson, Broad Institute of MIT and Harvard
Biomarker Discovery in Neuroimmune Diseases
Learn strategies to identify and validate molecular, cellular, and fluid-based biomarkers that link neuroimmune mechanisms to CNS disease outcomes and enhance translational relevance.
Workshop Lead: Michael Levy, Massachusetts General Hospital
The Role of Inflammation in the Biology of Mood Disorders
Examine how chronic inflammation and glial dysfunction contribute to depression and mood disorders, opening opportunities for biologically targeted therapies and patient stratification.
Workshop Lead: Jagadeesh Rao, IGC PHARMA; Matthew L Baum, Brigham and Women’s Hospital
Preclinical Strategy: In Vitro Models for Neuroimmunology
Explore how patient-derived and cell-based in vitro systems can accelerate proof-of-concept studies, bridge preclinical and clinical evidence, and reduce reliance on animal models.
Workshop Lead: Kevin Dines, Bristol Myers Squibb
Attending Companies Include